byū/spī’ rōn
Brand Names: BuSpar®
- Generic Available
Common Dosage Forms:
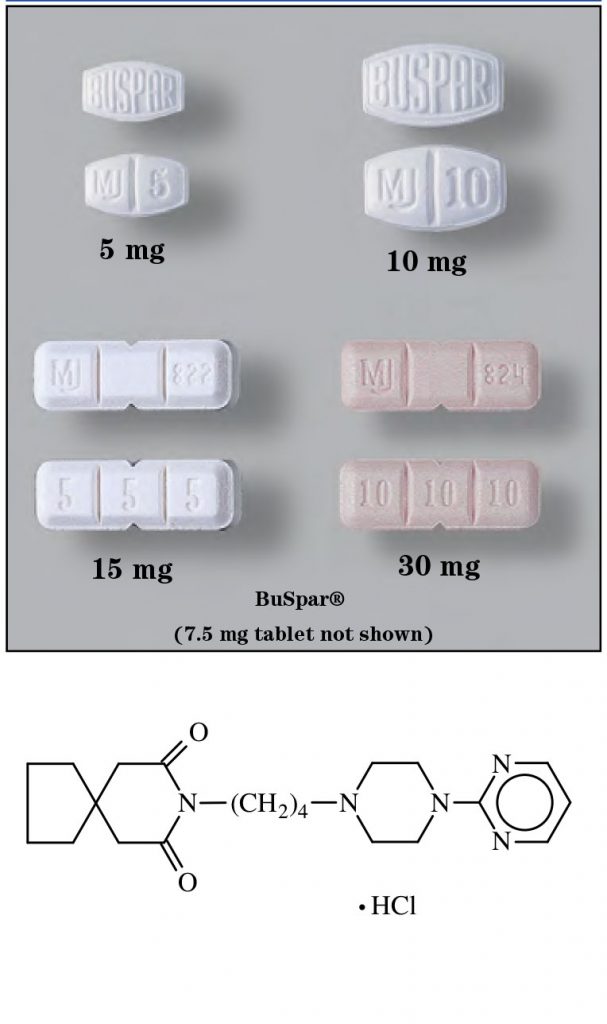
- Tablets: 5 mg (scored), 7.5 mg (scored), 10 mg (scored), 15 mg (tri-scored), and 30 mg (tri-scored)
FDA Indications/Dosages:
- Short-term relief of the symptoms of anxiety:
The recommended initial dose is 15 mg daily given in two divided doses. Doses may be increased in 5 mg increments at 2 to 3 day intervals to reach the optimum therapeutic response. Normal adult dose is 5 to 10 mg given up to four times a day.
Pharmacology/Pharmacokinetics:
The exact mechanism of action of buspirone in vivo is not known. It may be due to a number of changes in brain chemistry including; (1) increasing the metabolism of norepinephrine in the locus ceruleus, (2) acting as a moderate presynaptic dopamine agonist, and (3) acting as a serotonin agonist. Buspirone does not significantly affect benzodiazepine or GABA receptors. Therapeutic improvement is usually seen within 7 days but may take as long as 21 days. Although indicated for short-term relief of anxiety, some patients have received up to 20 mg per day for as long as one year without altering efficacy or side effects. Discontinuation of therapy does not cause withdrawal symptoms. Peak plasma levels are reached in 1-2 hours. Elimination half-life is between 2-3 hours. Metabolism occurs in the liver and excretion primarily in the urine.
Drug Interactions:
Coadministration with MONOAMINE OXIDASE INHIBITORS may elevate blood pressure. Do not use together. Grapefruit juice may increase plasma levels and should be avoided.
Contraindications/Precautions:
Use with caution in patients with moderate to severely impaired hepatic or renal function. Because of buspirone’s ability to bind to dopamine receptors, there is a possibility of dystonia, parkinsonism, and tardive dyskinesia occurring during therapy. Use with caution during lactation. Pregnancy Category B.
Adverse Effects:
Skin rash, tachycardia, headache, fatigue, sweating, muscle pain, dream disturbances, dizziness, drowsiness, nervousness, insomnia, mood alterations, depression, dry mouth, nausea, vomiting, diarrhea or constipation, nasal congestion, blurred vision, tinnitus, and sore throat.
Patient Consultation:
- May cause drowsiness or dizziness. Use caution when operating machinery or an automobile.
- Report to your physician or pharmacist any abnormal involuntary movements of the tongue or facial muscles and any change in motor restlessness.
- Store in a cool, dry place away from sunlight and children.
- Contact a physician if the above side effects are severe or persistent.
- If a dose is missed, skip it and return to normal dosing schedule.
- Avoid grapefruit and grapefruit juice during therapy.