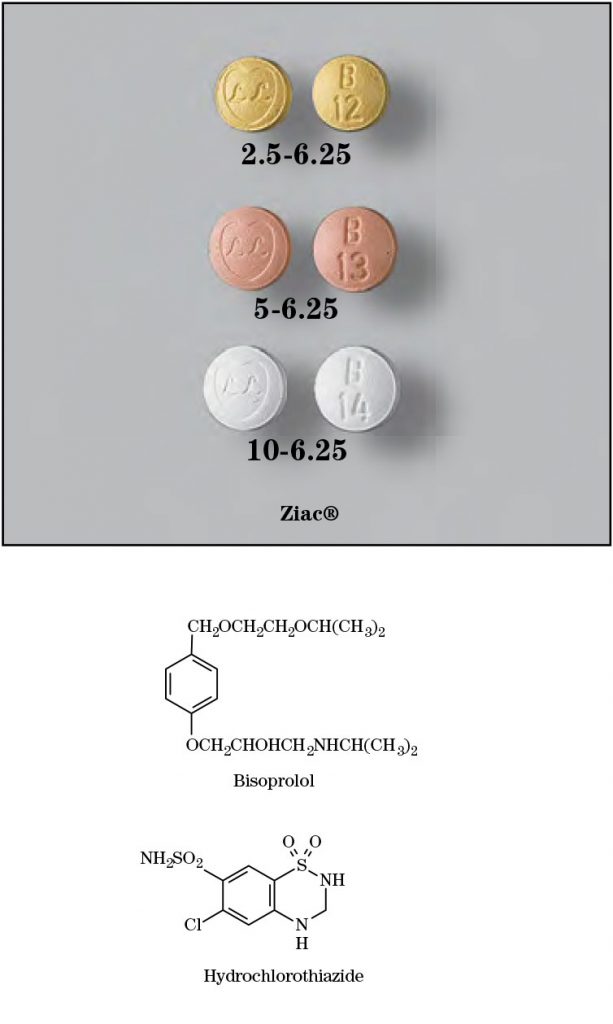
bis/ ō’ prō/lol hī’ drō/klōr/ō/thī’ ə/zīd
Brand Names: Ziac®
- Generic Available
Common Dosage Forms:
- 2.5/6.25 tablets: Each tablet contains bisoprolol fumarate 2.5 mg and hydrochlorothiazide 6.25 mg
- 5/6.25 tablets: Each tablet contains bisoprolol fumarate 5 mg and hydrochlorothiazide 6.25 mg
- 10/6.25 tablets: Each tablet contains bisoprolol fumarate 10 mg and hydrochlorothiazide 6.25 mg
FDA Indications/Dosages:
- For the management of hypertension: Initiate therapy with one 2.5/6.25 tablet given once daily. Dosage titration should occur after 14 day intervals up to a maximum daily dosage of two 10/6.25 tablets. The usual dose is one tablet daily in the morning. If cessation of therapy is desired, decrease dosage over a 2 week period while closely observing the patient.
Monitor:
BP
Pharmacology/Pharmacokinetics:
Bisoprolol is a beta-1-selective adrenergic blocking agent without significant membrane stabilizing or intrinsic sympathomimetic activities in its therapeutic dosage range. Although it is cardioselective, at high doses it possesses beta-2-adrenergic blocking properties. Its mechanism of action may be due to a decrease in cardiac output, an inhibition of renin release by the kidneys, an inhibition of tonic sympathetic outflow from vasomotor centers in the brain, or a combination of each. Bisoprolol reaches peak plasma levels in 3 hours after an oral dose with a terminal half ranging from 7 to 15 hours. 55% of an oral dose is recovered unchanged in the urine. Hydrochlorothiazide inhibits the reabsorption of sodium and chloride at the distal renal tubule. It reaches peak plasma levels in 4 hours, with a half-life of 6-15 hours. Excretion of unchanged drug occurs in the urine.
Drug Interactions:
Other antihypertensive agents may potentiate the actions of this agent. Use caution when using with clonidine, verapamil, and diltiazem. May increase the therapeutic and toxic effects of lithium. Absorption of hydrochlorothiazide may be impaired by cholestryramine or colestipol. Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
Contraindications/Precautions:
Contraindicated in patients in cardiogenic shock, overt cardiac failure, second or third degree AV block, marked sinus bradycardia, or anuria. Use with caution for inhalation in patients with compensated cardiac failure, peripheral vascular disease, bronchospastic pulmonary disease, diabetes, impaired renal function, impaired hepatic function, and during anesthesia. Measure serum electrolytes during therapy. Pregnancy Category C.
Adverse Effects:
The most common adverse effects are fatigue (3%), headache (4%), dizziness (5%), and diarrhea (4%).
Patient Consultation:
- May be taken with food or milk if GI upset occurs.
- Avoid abrupt withdrawal from therapy.
- Patients with diabetes need to be aware that this agent can cause masking of hypoglycemic symptoms.
- May cause dizziness. Use care when operating machinery or when mental alertness is required.
- Usually given in early a.m. due to increased diuresis.
- Avoid excessive exposure to sunlight.
- Store in a cool, dry place away from children.
- If a dose is missed take it as soon as possible. If it is closer to the time of your next dose than the dose you missed, skip the missed dose and return to your dosing schedule. Do not double doses.
- Contact a physician if the above side effects are severe or persistent or if you experience trouble in breathing or bradycardia.