ə/lin’ drō/nāt
Brand Names: Fosamax®
- Generic Available
Common Dosage Forms:
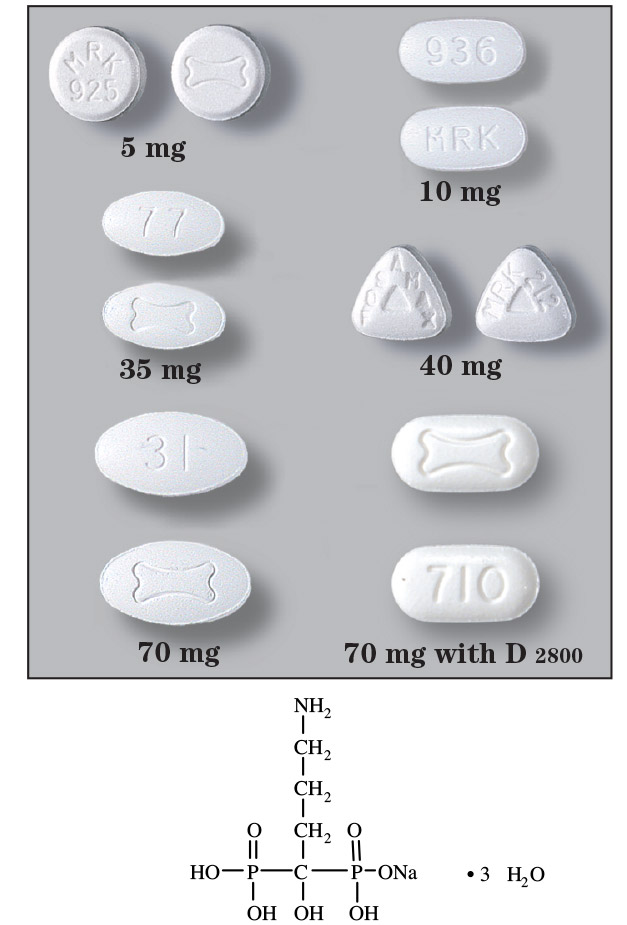
- Tablets: 5 mg, 10 mg, 35 mg, 40 mg and 70 mg
- Oral Solution: 70 mg/75 mL
- Fosamax Plus D 70/2800: Each tablet contains the equivalent of 70 mg alendronate free base and cholecalciferol equivalent to 2800 IU Vitamin D.
- Fosamax Plus D 70/5600: Each tablet contains the equivalent of 70 mg alendronate free base and cholecalciferol equivalent to 5600 IU Vitamin D.
FDA Indications/Dosages:
- For the treatment of osteoporosis in postmenopausal women (confirmed by the finding of low bone mass or by the presence or history of osteoporotic fracture): 10 mg once a day or 70 mg once a week.
- For the prevention of osteoporosis in postmenopausal women: 5 mg once a day or 35 mg once a week.
- To increase bone mass in men with osteoporosis: 10 mg once a day.
- Treatment of glucocorticoid-induced osteoporosis in men and women: 5 mg once a day except in postmenopausal women not receiving estrogen for whom the dose is 10 mg once a day.
- For the treatment of Paget’s disease of bone (confirmed by having alkaline phosphatase at least two times the upper limit of normal, in those who are symptomatic, or in those at risk for future complications from their disease): 40 mg once a day for six months.
- Doses must be swallowed whole with plain water immediately upon arising for the day (at least 30 minutes before the first food, beverage, or medication for the day). Patients should not lie down for 30 minutes after swallowing tablets and not until the first food of the day has been consumed.
Monitor: BMD
Pharmacology/Pharmacokinetics:
Alendronate adheres to sites of bone resorption inhibiting osteoclast activity. Normal bone is formed on top of alendronate which is incorporated into the bone matrix. Once in the matrix, alendronate is not pharmacologically active, therefore it must be continuously administered to repeat the process. The net effect is a decrease in bone turnover, bone formation exceeding bone resorption, and gains in bone mass. Vitamin D is required for normal bone formation and is involved in the dietary absorption of calcium and phosphate. After absorption, alendronate is rapidly distributed to bone or excreted in the urine unmetabolized.
Drug Interactions:
Calcium and antacids may decrease absorption if taken within 30 minutes of dose. Concomitant aspirin therapy may increase GI adverse effects. As a general rule, do not administer any oral medication within 30 minutes of alendronate.
Contraindications/Precautions:
Contraindicated in patients with abnormalities of the esophagus which delay esophageal emptying such as stricture or achalasia, in patients who cannot sit upright or stand for at least 30 minutes, and in patients with hypocalcemia. Alendronate may cause severe local irritation of the upper GI mucosa and patients should report any problems such as dysphagia, odynophagia, or retrosternal pain. Use with caution in patients with renal disease. Do not use when creatinine clearance <35mL/min. Do not use in nursing mothers. Pregnancy Category C.
Adverse Effects:
Adverse effects are rare and include abdominal pain, constipation, diarrhea, flatulence, and musculoskeletal pain. Rare cases of severe local irritation of the upper GI mucosa and focal osteonecrosis have occurred.
Patient Consultation:
- Doses must be swallowed with 6-8 ounces of plain water immediately upon arising for the day (at least 30 minutes before the first food, beverage, or medication for the day). Do not lie down for 30 minutes after swallowing the tablet and not until the first food of the day has been consumed.
- Promptly report any upper GI distress to your physician or pharmacist.
- Swallow whole; do not crush, chew or suck on the tablet.
- If a dose is missed, skip it and return to your normal schedule. Do not double doses.
- Contact a physician if the above side effects are severe or persistent.
- Store in a cool, dry place away from sunlight and children.