byū/prō’ pē/on
Brand Names: Wellbutrin®,Forfivo®XL,Zyban®^
- Generic Available
Common Dosage Forms:
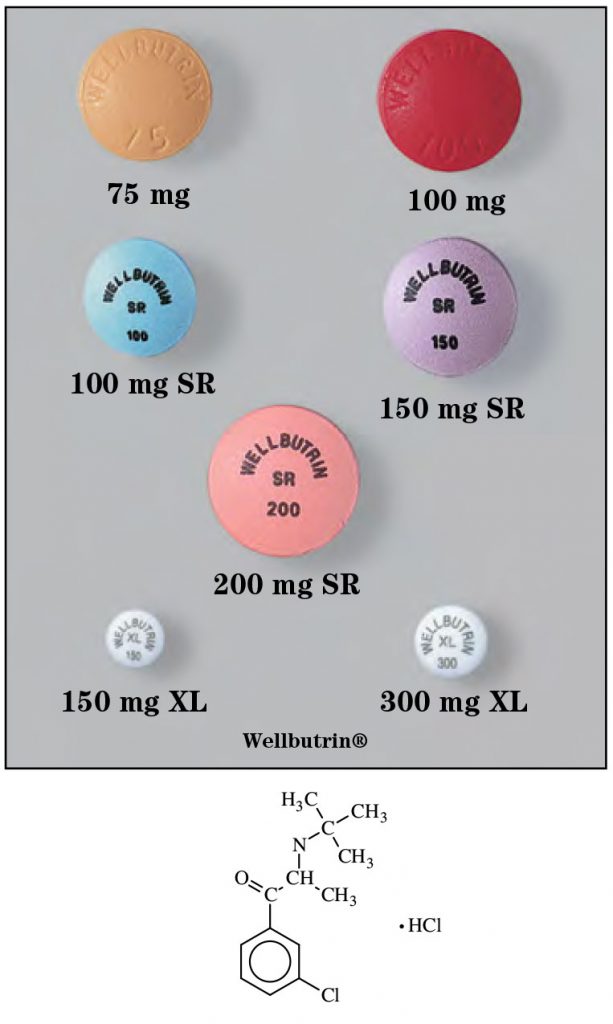
- Tablets, immediate-release: 75 mg, 100 mg
- Tablets, sustained-release (SR): 100 mg, 150 mg, 200 mg
- Tablets, extended-release (XL): 150 mg, 300 mg, 450 mg
- Tablets, sustained-release (Zyban): 150 mg
FDA Indications/Dosages:
- Treatment of depression and for the prevention of seasonal
major depressive episodes in patients with seasonal
affective disorder:
Immediate-release formulation: Start with 100 mg twice a day. Increase dose a maximum of 100 mg/day no sooner than every three days to achieve maximum benefit. Usual adult dose is 100 mg three times a day. Maximum daily dose is 450 mg/day. No single dose should exceed 150 mg. Use the lowest dose possible to achieve desired results. Sustained release formulation: Give up to 400 mg/day in two divided doses. Target dose is 300 mg/day. Extended-release formulation: 150 to 300 mg given once per day. - As an aid to smoking cessation (Zyban): Start with 150 mg daily for 3 days then increase to 150 mg twice a day.
Pharmacology/Pharmacokinetics:
Bupropion is unrelated to tricyclic or tetracyclic antidepressants. It is chemically related to phenylethylamines. Its actions seem to be produced by a dose-related CNS stimulant effect. It is a weak inhibitor of neuronal uptake of norepinephrine and dopamine, and does not inhibit monoamine oxidase. Its effects are thought to be mediated by noradrenergic and/or dopaminergic mechanisms. Peak steady-state plasma levels following BID dosing of sustained- release formulations are 15% lower and trough concentrations are 7% higher than those found after the immediate- release formulation is given TID. Plasma half-life is between 8 to 24 hours. Bupropion is metabolized to inactive and active metabolites by the CYP2B6 isoenzyme system and excreted in the urine and feces. Up to 80% is bound to plasma proteins.
Drug Interactions:
Toxicity is increased by monoamine oxidase inhibitors and L-dopa. Alcohol may lower seizure threshold. Drugs which affect the CYP2B6 isoenzyme system (orphenadrine, thiotepa, cyclophosphamide, ticlopidine) may affect bupropion plasma levels. Ritonavir and lopinavir reduce the plasma levels of bupropion. Bupropion is an inhibitor of CYP2D6 therefore it may increase plasma levels of tricyclic antidepressants, paroxetine, fluoxetine, sertraline, haloperidol, risperidone, thioridazine, metoprolol, propafenone, or flecainide.
Contraindications/Precautions:
Contraindicated in patients with a seizure disorder, history of bulimia or anorexia nervosa, or with 14 days of therapy with a monoamine oxidase inhibitor. ANTIDEPRESSANTS INCREASE SUICIDAL THOUGHTS AND ACTIONS IN CHILDREN, ADOLESCENTS, AND YOUNG ADULTS. Wellbutrin has been associated with precipitating dose-related seizures. Seizure incidence increases tenfold between daily doses of 450 mg and 600 mg. May cause various psychomotor disturbances such as mania, psychosis, and hallucinations. Use with caution in patients with impaired liver or kidney function. Pregnancy Category C.
Adverse Effects:
Dizziness, constipation, weight loss, dry mouth, insomnia, sweating, agitation, and tremor.
Patient Consultation:
- Take at immediate-release tablets at regular intervals no sooner than three to four times a day.
- Take extended-release tablets in the morning if possible to avoid insomnia.
- Swallow SR and XL tablets whole–do not crush or chew.
- If a dose is missed, wait until the next scheduled dose–DO NOT DOUBLE DOSES.
- Do not take OTC medications without first consulting a physician or pharmacist.
- Refrain from consuming alcohol.
- Store in a cool, dry place away from sunlight.
- Contact a physician if side effects become severe or bothersome or if a seizure occurs.
- Weight loss or gain may be temporary–contact your physician if bothersome.
- Pay close attention to any changes, especially sudden changes, in mood behaviors, thoughts, or feelings. Antidepressants may increase suicidal thoughts or actions.
- Continued therapy of up to 2 weeks may be needed to show noticeable improvement.