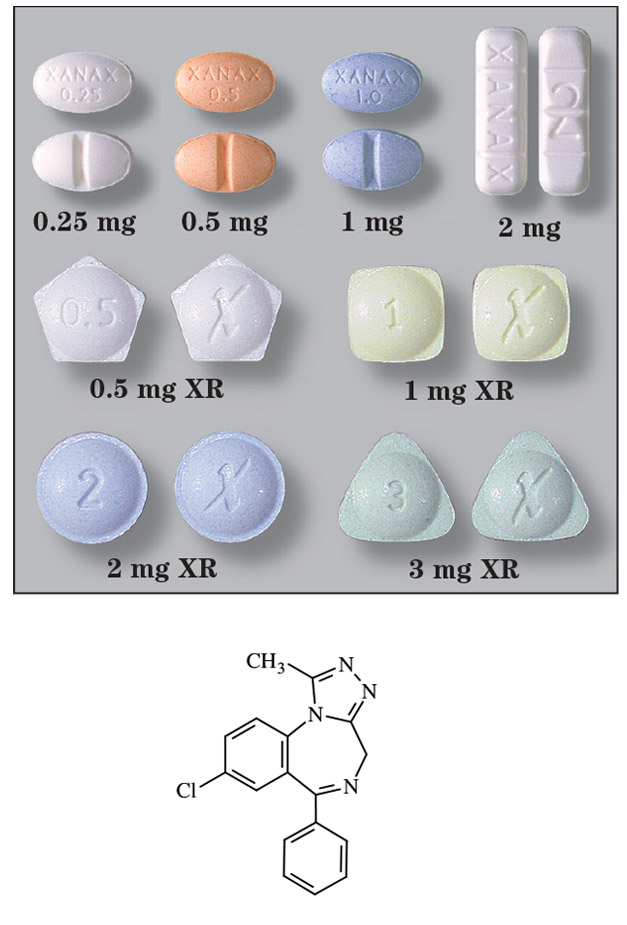
al/praz’ ō/lam
Brand Names: Xanax®(XR)
- Generic Available
Common Dosage Forms:
- Tablets, immediate release: 0.25 mg, 0.5 mg, 1 mg, and 2 mg
- Tablets, extended release: 0.5 mg, 1 mg, 2 mg, and 3 mg
- Tablets, orally-disintegrating: 0.25 mg, 0.5 mg, 1 mg, and 2 mg
- Oral Solution Concentrate: 1 mg/mL
FDA Indications/Dosages:
- Management of anxiety disorders or for the short-term relief of the symptoms of anxiety or anxiety-associated depressive states (immediate release tablets)*: 0.25 mg to 0.5 mg initially, given three times a day. Titrate dose to individual needs. May take up to 4 mg a day in divided doses.
- Treatment of panic disorder (immediate release tablets)*: Initial dose is 0.5 mg three times a day. Depending on the response, the dose may be increased at intervals of 3 to 4 days in increments of no more than 1 mg/day.
- Treatment of panic disorder, with or without agoraphobia (extended release tablets)*: One tablet daily, preferably in the morning. Initiate dose with 0.5 mg to 1 mg. Doses may be increased in 1 mg increments every 3 to 4 days. The recommended total daily dose ranges between 3 to 6 mg/day. Elderly patients should be initiated at 0.5 mg per day.
*Therapy should not be discontinued abruptly. Reduce dosage by 0.5 mg every 3 to 4 days.
Pharmacology/Pharmacokinetics:
Benzodiazepines facilitate the inhibitory neuro- transmitter action of gamma-aminobutyric acid (GABA), which mediates both pre- and post- synaptic inhibition in all regions of the CNS. Alprazolam reaches a peak plasma level in humans in approximately 1 to 2 hours. The mean elimination half-life is 11.2 hours. Excretion of unchanged and metabolized drug occurs in the urine. Alprazolam is metabolized in the liver and is a known substrate for cytochrome P450 (CYP) isoenzyme 3A4. Drugs that inhibit the CYP3A4 isoenzyme may have a profound affect on the clearance of alprazolam. XR tablet kinetics are similar to immediate-release tablets with the exception of a slower rate of absorption. The slower rate of absorption results in a relatively constant concentration that is maintained between 5 and 11 hours after the dosing.
Drug Interactions:
CNS depression is increased when used with other CNS depressants. Oral contraceptives can increase the effects of alprazolam
Contraindications/Precautions:
Contraindicated in patients hypersensitive to benzodiazepines or in patients with narrow-angle glaucoma. CONCOMITANT USE WITH OPIOIDS INCREASES THE RISK OF RESPIRATORY DEPRESSION, COMA, AND DEATH. Use with caution in patients with primary depression, psychosis, and impaired renal or hepatic function. Withdrawal symptoms can be serious, especially in patients on high doses or extended therapy (>8-12 weeks). Use only during pregnancy or lactation when benefits clearly outweigh risks. Pregnancy Category D.
Adverse Effects:
Drowsiness and hypotension.
Patient Consultation:
- May cause drowsiness. Use caution while operating machinery or when mental alertness is required.
- Avoid alcohol while taking this medication.
- WARNING: This medication may be habit- forming.
- Store in a cool, dry place away from sunlight and children.
- Contact a physician if side effects are severe or persistent.
- If a dose is missed, skip it and return to normal dosing schedule.
- XR tablets should not be discontinued abruptly.
- XR tablets should be swallowed whole – do not crush, chew, or break.
- Therapy should not be discontinued abruptly. Reduce dosage by 0.5 mg every 3 to 4 days.