Rank#: 166
Brand Name: Lotrel®
• Generic AvailableCommon Dosage Forms:
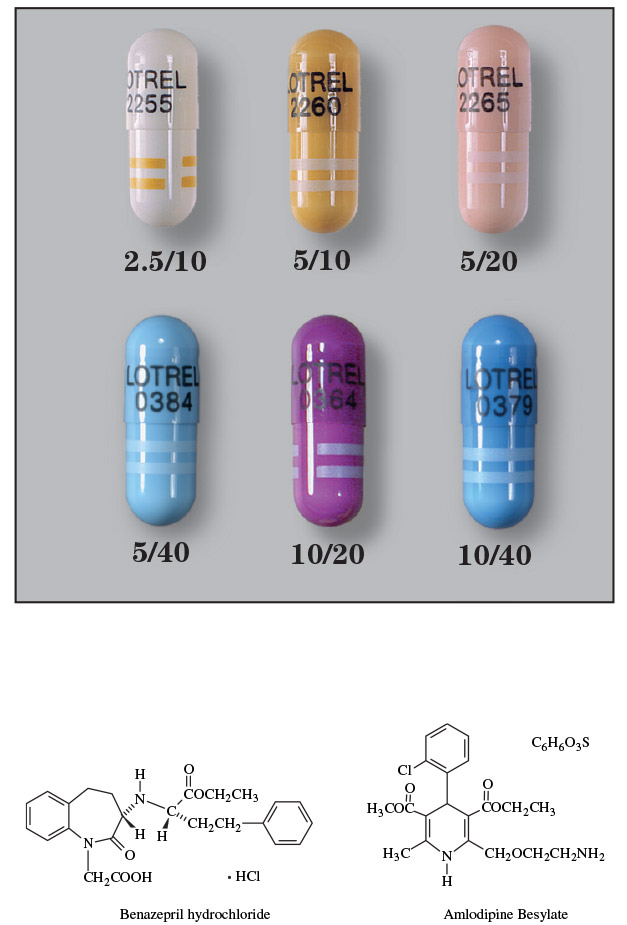
- Lotrel 2.5/10: Each capsule contains 2.5 mg amlodipine and 10 mg benazepril
- Lotrel 5/10: Each capsule contains 5 mg amlodipine and 10 mg benazepril
- Lotrel 5/20: Each capsule contains 5 mg amlodipine and 20 mg benazepril
- Lotrel 5/40: Each capsule contains 5 mg amlodipine and 40 mg benazepril
- Lotrel 10/20: Each capsule contains 10 mg amlodipine and 20 mg benazepril
- Lotrel 10/40: Each capsule contains 10 mg amlodipine and 40 mg benazepril
FDA Indications/Dosages:
- Treatment of hypertension: One capsule daily. The lowest dose of amlodipine is favorable to avoid peripheral edema. Use this combination in patients who failed to achieve desired effects with one or the other monotherapy or in patients who failed to achieve desired effects with amlodipine without developing edema.
Monitor:
K, BP, CrCl, WBC
Pharmacology/Pharmacokinetics:
Amlodipine inhibits the movement (influx) of calcium ions across specific cellular membranes (slow channels) in vascular smooth muscle and cardiac muscle. Amlodipine inhibits calcium influx in vascular smooth muscle more than in cardiac muscle. Specific effects include: (1) a decrease in peripheral vascular resistance; (2) a dilation of coronary arteries and arterioles; (3) an inhibition of coro- nary spasm. Benazepril ‘s mechanism of action is thought to be due to its suppression of the renin-angiotensin-aldos- terone system by competitively inhibiting the angiotensin- converting enzyme (ACE). Inhibition of ACE decreases plasma angiotensin II which leads to decreased aldosterone secretion. The net result is a decrease in peripheral arterial resistance. Benazepril is metabolized to the more active benazeprilat in the liver. Peak plasma levels are reached in 6-12 hours. Amlodipine is metabolized in the liver and excreted in the urine as inactive metabolites.
Drug Interactions:
Indomethacin may decrease therapeutic effects. Capsaicin may increase the incidence of coughing. Because of potas- sium-sparing effect, use caution when given with potas- sium-sparing diuretics and potassium supplements. Lithium toxicity may occur. Amlodipine may increase the plasma levels of simvastatin. Increased adverse effects may occur when used with other renin-angiotensin system blockers or aliskiren.
Contraindications/Precautions:
DO NOT USE DURING PREGNANCY. Use with caution in patients with impaired renal or hepatic function, collagen vascular disease (may cause agranulocytosis), or aortic stenosis, and in patients undergoing surgery or anesthesia. Patients should report to their physician any sign of facial swelling, difficulty in breathing, or infection (unex- plained fever, sore throat). May cause rare increase in angina or myocardial infarction on starting therapy or increasing dose. Use with caution in congestive heart failure. The maximum dose of simvastatin is 20 mg daily when used with amlodipine. Pregnancy Category D.
Adverse Effects:
The most frequent adverse effects include cough (3%), headache, dizziness, and edema (2%), nausea, flushing, fatigue, and abdominal pain (<1%). A potentially severe adverse effect is angioedema.
Patient Consultation:
- May be taken without regard to meals.
- Do not discontinue medication unless otherwise directed by your physician.
- Avoid nonprescription cough, cold, and allergy medications unless otherwise directed.
- Avoid salt substitutes containing potassium.
- Report any sign of facial swelling, difficulty in breathing, or infection (fever, sore throat) to your physician.
- Store in a cool, dry place away from sunlight and children.
- If a dose is missed, take it as soon as possible. If it is closer to the time of your next dose than the dose you missed, skip the missed dose and return to your dosing schedule. Do not double doses.