am/fet’ ə/mēn with
deks’ trō/am/fet’ ə/mēn
Brand Name: Adderall®, Adderall XR
• Generic AvailableCommon Dosage Forms:
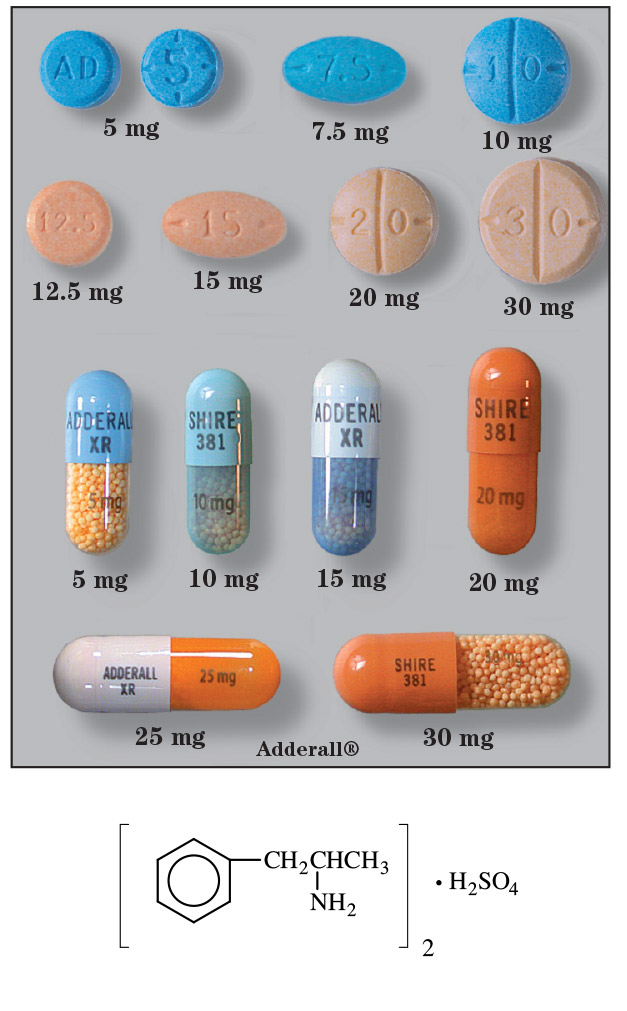
- Tablets (Each tablet contains equal amounts of dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate): 5 mg, 7.5 mg, 10 mg, 12.5 mg, 15 mg, 20 mg, and 30 mg.
- Capsules, extended-release (Each capsule contains equal amounts of dextroamphetamine saccharate, amphetamine aspartate, dextroamphetamine sulfate, and amphetamine sulfate): 5 mg, 10 mg, 15 mg, 20 mg, 25 mg and 30 mg.
FDA Indications/Dosages:
- Treatment of the symptoms of narcolepsy (tablets): Usual dose is 5 to 60 mg per day in divided doses.
- Treatment of Attention Deficit Disorder with Hyperactivity: Children from 3 to 5 years of age: Start with 2.5 mg daily and increase by 2.5 mg at weekly intervals until optimum effect is seen. Children over 5 years of age: Start with 5 to 10 mg daily and increase by 5 mg at weekly intervals until optimum effect is seen. XR capsules can be substituted for immediate-release tablets at the same total daily dose taken once daily. XR capsules can be swallowed whole or the capsule carefully opened and the contents sprinkled on applesauce. The applesauce/drug mixture should be immediately swallowed whole without chewing Adults (XR Capsules): The recommended dosage is 20 mg per day.
Pharmacology/Pharmacokinetics:
Amphetamine’s mechanism of action in Attention Deficit Disorder is unknown. In adults it causes CNS stimulation by a direct action on adrenergic receptors, releasing norepinephrine from storage sites. Immediate- release tablets reach maximum plasma levels in 3 hours and extended-release capsules reach Cmax in 7 hours.
Drug Interactions:
Effects may be increased when used with MONOAMINE OXIDASE INHIBITORS and furazolidone. Urine alkalinizing agents (sodium bicarbonate) increase reabsorption while GI alkalinizing agents increase absorption. May decrease effects of antihypertensives.
Contraindications/Precautions:
Contraindicated in advanced arteriosclerosis, cardiovascular disease, moderate hypertension, hyperthyroidism, agitated states, glaucoma, and within 14 days of therapy with monoamine oxidase inhibitors. CNS STIMULANTS HAVE A HIGH POTENTIAL FOR ABUSE AND DEPENDENCE. Do not use in patients with a known history of drug abuse. Use with caution in patients with mild hypertension. Pregnancy Category C.
Adverse Effects:
Restlessness, dizziness, insomnia, euphoria, tremor, headache, palpitations, tachycardia, dry mouth, unpleasant taste, diarrhea or constipation, and anorexia.
Patient Consultation:
- May cause dizziness – use caution when performing activities that require coordination.
- Do not exceed prescribed dosage.
- This prescription cannot be refilled.
- WARNING – this medication may be habit- forming.
- Store in a cool, dry place away from sunlight and children.
- Contact a physician if the above side effects are severe or persistent.
- If a dose is missed, skip it and return to normal dosing schedule.
- XR capsules can be swallowed whole or the capsule carefully opened and the contents sprinkled on applesause. The applesauce/drug mixture should be immediately swallowed whole without chewing.